- News & information
- About
- History
- George V. Voinovich
- George V. Voinovich Collection
- Calendar
- How to Find Us
- News
- Archives
- Photojournalism Fellowship Project
- Photo Essays
- Current Fellow
- Previous Fellows
- Reports and Publications
- Archives
- Students
- Prospective
- Center for Entrepreneurship
- Environmental Studies
- HTC/Voinovich School Scholars
- Master of Public Administration
- Current
- HTC/Voinovich School Scholars
- Center for Entrepreneurship
- Environmental Studies
- Master of Public Administration
- Alumni
- Contact
- School Leadership
- Strategic Partners Alliance
- Ohio University Public Affairs Advisory Committee
- Ohio University Public Affairs Advisory Committee
- Faculty and Fellows
- Faculty
- Visiting Professors
- Voinovich Fellows
- Professional Staff
Chapter 9: Carbon Dioxide (R744) - The New Refrigerant
Introduction and Discussion
In the early days of refrigeration the two refrigerants in common use were ammonia and carbon dioxide. Both were problematic - ammonia is toxic and carbon dioxide requires extremely high presures (from around 30 to 200 atmospheres!) to operate in a refrigeration cycle, and since it operates on a transcritical cycle the compressor outlet temperature is extremely high (around 160°C). When Freon 12 (dichloro-diflouro-methane) was discovered it totally took over as the refrigerant of choice. It is an extremely stable, non toxic fluid, which does not interact with the compressor lubricant, and operates at pressures always somewhat higher than atmospheric, so that if any leakage occured, air would not leak into the system, thus one could recharge without having to apply vacuum.
Unfortunately when the refrigerant does ultimately leak and make its way up to the ozone layer the ultraviolet radiation breaks up the molecule releasing the highly active chlorine radicals, which help to deplete the ozone layer. Freon 12 has since been banned from usage on a global scale, and has been essentially replaced by chlorine free R134a (tetraflouro-ethane) - not as stable as Freon 12, however it does not have ozone depletion characteristics.
Recently, however, the international scientific consensus is that Global Warming is caused by human energy related activity, and various man made substances are defined on the basis of a Global Warming Potential (GWP) with reference to carbon dioxide (GWP = 1). R134a has been found to have a GWP of 1300 and in Europe, within a few years, automobile air conditioning systems will be barred from using R134a as a refrigerant.
The new hot topic is a return to carbon dioxide as a
refrigerant. The previous two major problems of high pressure and
high compressor temperature are found in fact to be advantageous. The
very high cycle pressure results in a high fluid density throughout
the cycle, allowing miniturization of the systems for the same heat
pumping power requirements. Furthermore the high outlet temperature
will allow instant defrosting of automobile windshields (we don't
have to wait until the car engine warms up) and can be used for
combined space heating and hot water heating in home usage.
Refer
to the following relevant web resources:
Air Conditioning Heating
and Refrigeration (ACHR) News:
CO2
as Refrigerant: The Transcritical Cycle
The
ubiquitous Wikipedia:
Sustainable
automotive air conditioning
Building
Green:
A
Heat Pump Using Carbon Dioxide as the Refrigerant
Emerson
Climate Conversation:
CO2
as a Refrigerant
(Includes
a series of 13 posts)
Danfoss:
Natural
Refrigerants – CO2
Environmental
Leader:
Automakers
Develop CO2-Based Air Conditioning
(including
Volkswagen, Daimler, Audi, BMW and Porsche)
Property Tables for Carbon Dioxide (R744)
We were not able to find any published tables for Carbon Dioxide (R744) refrigerant, hence decided to create our own. The following set of tables was developed using software from the NIST (National Institute for Standards and Technology) and has been organized in a format suitable for evaluating refrigeration and heat pump systems
Thermodynamic Properties of Carbon Dioxide R744
The P-h diagram for Carbon Dioxide (R744)
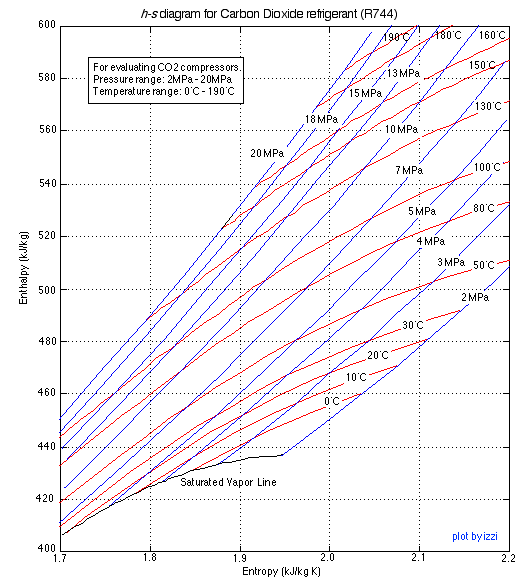
The h-s Diagram for Carbon Dioxide (R744)
A Heat Pump System using Carbon Dioxide Refrigerant (R744)
In addition to being a environmentally benign fluid, there can be significant advantages to using carbon dioxide in a home air-conditioning/heat-pump system environment. Consider the following system diagram:
Notice that in addition to serving as an air conditioner/space heater, the high compressor outlet temperature can be used to provide hot water at a significant economy over the regular gas or electric hot water heater. Thus the heat flow to the hot water heater cools the gas from 160°C to 70°C, and the heat flow to the space heater further reduces the gas temperature to 45°C.
In order to determine the enthalpy at outlet station (4) we need to consider the energy equation applied to the internal heat exchanger. Since we assume that it is externally adiabatic, all the heat transfer is internal, as shown in the following:
Drawing all the processes of the above scheme on a P-h diagram we obtain the following diagram. Notice the sketches on the diagram of the various components as well as the internal heat exchanger indicating the heat flow from the gas cooler outlet (3) - (4) to the compressor inlet (6) - (1).

Problem 9.1 - Use the R744 refrigerant property tables in order to evaluate the following:
-
a) Determine the work done on the compressor [ 97 kJ/kg ].
-
b) Determine the heat rejected to the hot water heater [ 164 kJ/kg ], and that rejected to the space heater [ 97 kJ/kg ].
-
c) Determine the Coefficient of Performance of the hot water heater [ COP hw =1.7 ] and that of the space heater [ COP space =1 ] (Recall that COP is defined as the desired heat transferred divided by the work done on the compressor).
-
d) Determine the Coefficient of Performance of the air conditioner [ COP a/c =1.7 ]. (Notice from the P-h diagram that the internal heat exchanger significantly increases the capacity of the air conditioner.)
Problem 9.2 - For the following additional questions we can assume that the compressor power is 1kW. (Note - we can use the COP values to answer these questions - we do not need to evaluate the mass flow rate of the refrigerant ):
-
a) Determine how long it will take to heat 100 liters of water in the tank from 30°C to 60°C [ 2 hours ]
-
b) During the summer months when the air conditioner is operating, determine the volumetric flow rate of the air [ 5.1 m 3 /min ] flowing through the evaporator cooling duct in order to reduce the air temperature from 30°C to 13°C. (Note - assume a pressure of 100kPa and temperature of 25°C to evaluate the specific volume of the air)
-
c) During the winter months when the heat pump is operating, and using the same fan as above determine increase in temperature of the air [ 10°C ] flowing through the space heating duct.
Problem 9.3 - Notice that the compressor does not follow an isentropic process. This is a practical system with data adapted from a previous Visteon Corp. automobile a/c design. In this exercise you should do the following:
-
Plot the compressor process (1)-(2) on the enthalpy-entropy ( h-s ) diagram provided above. Plot also the isentropic compressor process and (using the Property Tables) determine the compressor isentropic efficiency η C . Recall that the compressor isentropic efficiency η C is defined as the isentropic work done on the compressor divided by the actual work done. Indicate both the isentropic work done and the actual work done on the h-s diagram. [ η C =74% ]
Problem 9.4 - A R744 (CO2) Home Geothermal Heat-Pump - It is a well known fact that there is a year round constant temperature only a few meters below the earths surface. In this problem we wish to evaluate a system which is designed to use this underground thermal source to advantage.

Engineering Thermodynamics by Israel
Urieli
is licensed under a Creative
Commons Attribution-Noncommercial-Share Alike 3.0 United States
License
Contact Information:
(740) 593–9381 | Building 21, The Ridges
Ohio University Contact Information:
Ohio University | Athens OH 45701 | 740.593.1000 ADA Compliance | © 2018 Ohio University . All rights reserved.



