- News & information
- About
- History
- George V. Voinovich
- George V. Voinovich Collection
- Calendar
- How to Find Us
- News
- Archives
- Photojournalism Fellowship Project
- Photo Essays
- Current Fellow
- Previous Fellows
- Reports and Publications
- Archives
- Students
- Prospective
- Center for Entrepreneurship
- Environmental Studies
- HTC/Voinovich School Scholars
- Master of Public Administration
- Current
- HTC/Voinovich School Scholars
- Center for Entrepreneurship
- Environmental Studies
- Master of Public Administration
- Alumni
- Contact
- School Leadership
- Strategic Partners Alliance
- Ohio University Public Affairs Advisory Committee
- Ohio University Public Affairs Advisory Committee
- Faculty and Fellows
- Faculty
- Visiting Professors
- Voinovich Fellows
- Professional Staff

Associate Professor of Cell Biology
Department of Biomedical Sciences
berryman@ohio.edu
237 Life Sciences
740-593-2364
| Click the images to enlarge. | ||
|
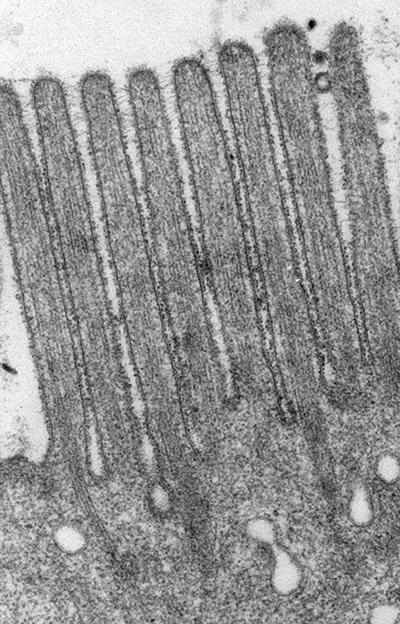
Figure 1:
Classic microvilli of the brush border that
lines the luminal surface of absorptive
epithelial cells. This image is from
neonatal rat intestine. Note the orderly
arrangement and uniform shape and size of
the microvilli. Bundles of parallel actin
filaments within each microvillus project
downward into the apical cytoplasm of the
cell.
( full image : 392kb) |
|
|
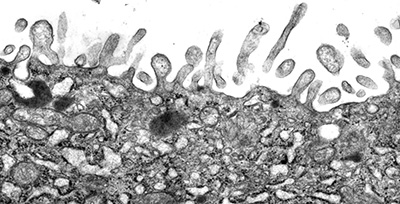
Figure 2:
Microvilli are not all created equal. Unlike
the intestinal brush border, microvilli
lining the apical surface of human placental
syncytiotrophoblast epithelium are variable
in distribution and shape. Presumably, these
microvilli are less rigid and more dynamic
than microvilli of the intestinal brush
border, perhaps reflecting active
endocytosis and membrane trafficking in
these cells. Placental microvilli contain
high levels of ezrin, a member of the ERM (Ezrin/Radixin/Moesin)
family of membrane-cytoskeletal crosslinking
proteins.
( full image : 4,532kb) |
|
|
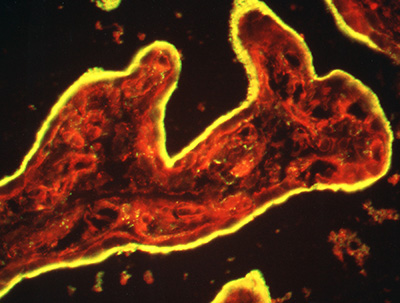
Figure 3:
Cryosection of intact human placenta tissue
stained for ezrin (green) and F-actin (red).
The syncytiotrophoblast epithelium is yellow
due to intense staining and colocalization
of ezrin and actin in surface microvilli.
( full image : 603kb) |
|
|
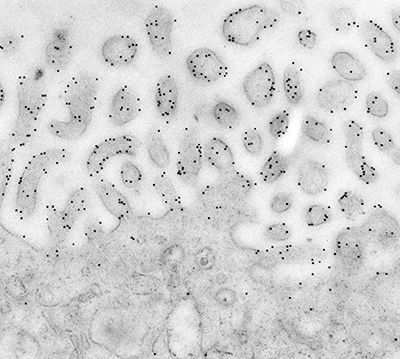
Figure 4:
Immunoelectron micrograph in which 10-nm
gold particles reveal the localization of
ezrin to microvilli in human placenta. In
many cases, gold particles are located on or
near the plasma membrane.
( full image : 447kb) |
|
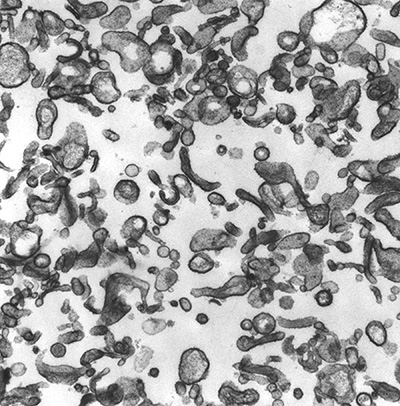
|
Figure 5:
Microvilli isolated from human placenta.
CLIC5A (Chloride Intracellular Channel 5A)
was biochemically isolated from this type of
preparation by virtue of its interaction
with ezrin and other actin-associated
proteins.
( full image : 664kb) |
|
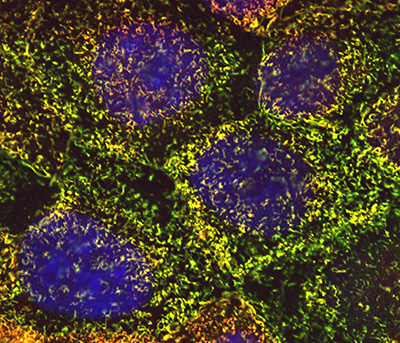
|
Figure 6:
Cultured human JEG-3 choriocarcinoma cells
stained for ezrin (green), CLIC5A (red), and
DNA (blue). The surface microvilli appear
yellow due to the colocalization of ezrin
and CLIC5A.
( full image : 1,287kb) |
|
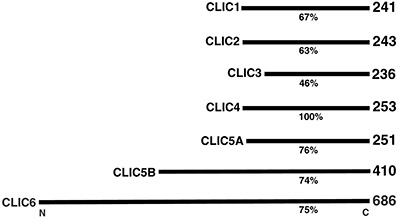
|
Figure 7:
Comparison of percent amino acid sequence
identity and number of amino acid residues
for members of the human CLIC (Chloride
Intracellular Channel) protein family, which
is comprised of six distinct genes. Gene
mutations that cause deficiencies of CLIC5A
can result in deafness and vertigo in humans
and mice.
( full image : 96kb) |
|
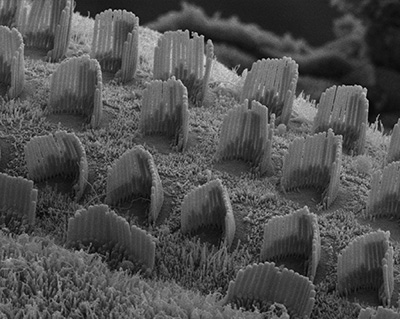
|
Figure 8:
Hearing takes place in the organ of Corti,
the sensorineural organ of the cochlea. It
consists of mechanosensory hair cells, nerve
fibers, and supporting structures. This
scanning electron micrograph shows the hair
bundles that project from the top surface of
each hair cell. Each hair bundle consists of
a highly organized group of stereocilia
(“giant microvilli”), pencil-shaped
protrusions containing a tightly packed
bundle of parallel actin filaments. There is
one row of inner hair cells and three rows
of outer hair cells. Each hair cell is
surrounded by support cells with smaller,
more typical microvilli, resembling “shag
carpet”. Credit: Leonardo Andrade
(collaborator) https://www.researchgate.net/profile/Leonardo_Andrade
( full image : 834kb) |
|
|
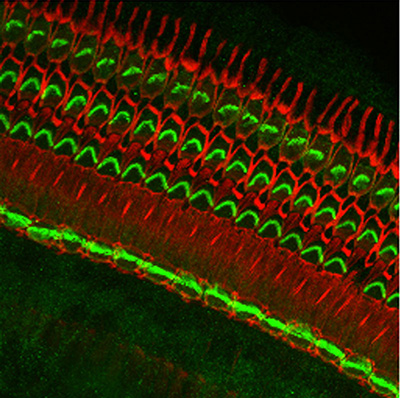
Figure 9:
Localization of CLIC5A (green) and actin
(red) in the organ of Corti from guinea pig
inner ear. CLIC5A is concentrated in hair
bundles that project from the apical surface
of hair cells, which function as
mechanosensory organelles in the auditory
and vestibular systems. CLIC5A staining is
prominent in the single row of inner hair
cells towards the bottom of the field, and
all three rows of outer hair cells in the
upper part of the field. The red staining is
primarily associated with the support cells
of the neurosensory epithelium.
( full image : 682kb) |
|
|
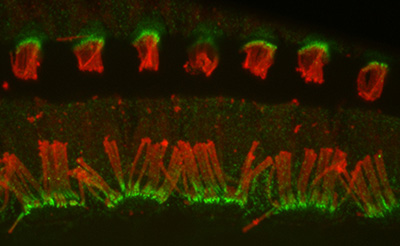
Figure 10:
Confocal image of CLIC5A (green) and β-actin
(red) in inner (bottom half) and outer
(upper half) hair cells from adult mouse.
The key point here is that CLIC5
concentrates at the base of stereocilia,
which is a distinct compartment with special
structural and functional significance.
Credit: Felipe Salles (collaborator) https://www.researchgate.net/profile/Felipe_Salles
( full image : 253kb) |
|
|
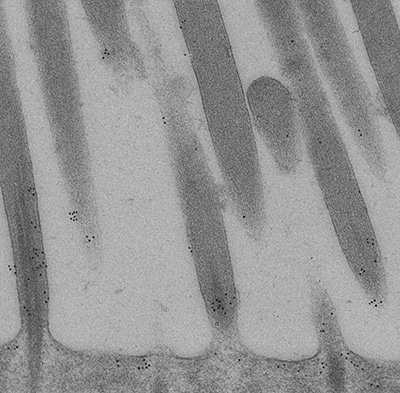
Figure 11:
High magnification immunoelectron micrograph
of CLIC5A stained with 10-nm gold particles.
This ultrathin section grazes through parts
of several stereocilia. Gold particles are
more plentiful near the base of stereocilia
(bottom of field), and are located near the
plasma membrane between stereocilia. Credit:
Felipe Salles (collaborator) https://www.researchgate.net/profile/Felipe_Salles
( full image : 1,431kb) |
|
|
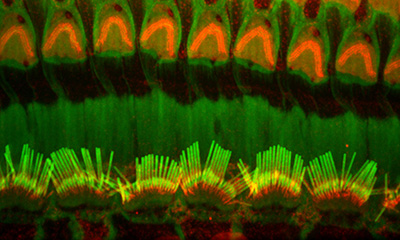
Figure 12:
Confocal image of Radixin (red) and F-actin
(green) in inner (bottom half) and outer
(upper half) hair cells from adult rat.
Radixin, another deafness-associated
protein, concentrates at the base of
stereocilia.
( full image : 404kb) |
|
|
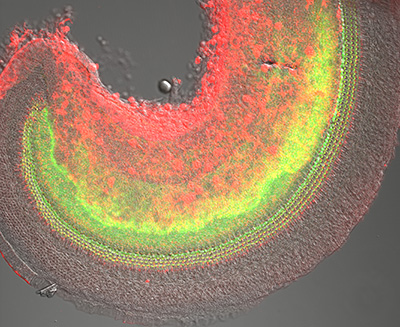
Figure 13:
Confocal overview of apical turn of neonatal
mouse cochlea stained for phosphorylated
Radixin (green) and β-actin (red):
superimposed on DIC image (gray). The
phosphorylated form of Radixin corresponds
to its functionally active conformation as a
membrane-cytoskeletal crosslinker. Credit:
Soichi Tanda (collaborator) https://www.researchgate.net/profile/Soichi_Tanda
( full image : 2,535kb) |
|
|
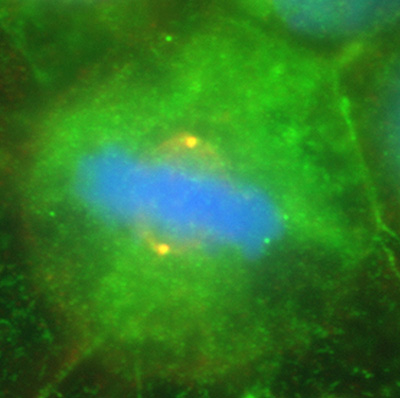
Figure 14:
Colocalization of CLIC4 (green) and γ-tubulin
(red) in a pair of centrosomes of a cultured
human JEG-3 choriocarcinoma cell in
metaphase: DNA (blue). The centrosomes
appear yellow due to the strong overlap in
staining of CLIC4 and γ-tubulin. This image
shows that in addition to actin-based
organelles, such as microvilli, CLIC
proteins can also associate with
microtubule-based cytoskeletal structures.
( full image : 225kb) |
|
|
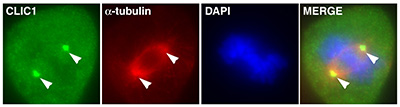
Figure 15:
Localization of CLIC1 (green) to poles of a
metaphase mitotic spindle (α-tubulin, red)
in a cultured NIH 3T3 fibroblast.
( full image : 230kb) |
|
|
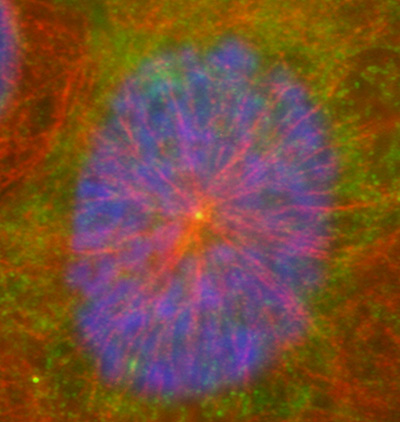
Figure 16:
Localization of CLIC4 (green) to the center
of a microtubule aster (red) in a cultured
human JEG-3 choriocarcinoma cell in
interphase: DNA (blue).
( full image : 278kb) |
|
|
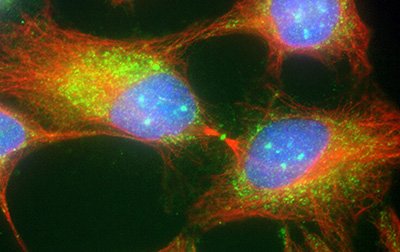
Figure 17:
Cytokinesis in cultured human JEG-3
choriocarcinoma cells stained with CLIC4
(green) and α-tubulin (red): DNA (blue).
CLIC4 is highly concentrated at the midbody,
the site of abscission that separates two
daughter cells during the final stages of
mitosis.
( full image : 2,283kb) |
|
|
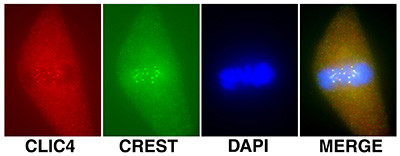
Figure 18:
Localization of CLIC4 (red) to kinetochores
identified by human CREST autoantibody
(green) during metaphase in a cultured
bovine aortic endothelial cells: DNA (blue).
Kinetochores consist of a large protein
complex associated with the centromere of a chromosome during cell
division, to which the mictotubules of the
mitotic spindle attach.
( full image : 178kb) |
|
|
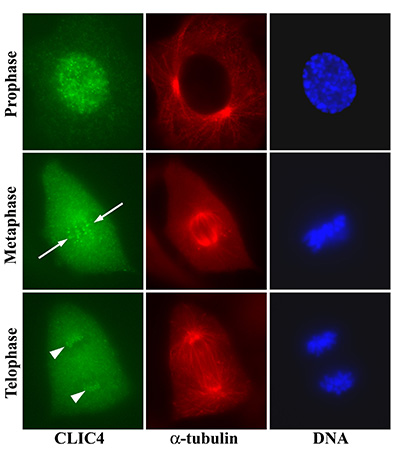
Figure 19:
Localization of CLIC4 to kinetochores of
bovine aortic endothelial cells in different
stages of mitosis. Discrete dots of CLIC4
first appear during prophase after
centrosome duplication and initiation of
chromosome condensation. In metaphase, the
CLIC4 dots align parallel to the long axis
of the mitotic spindle (arrows) and overlap
with the condensed chromosomes on the
metaphase plate. In telophase, the CLIC4
dots are clustered at opposite poles of the
spindle apparatus (arrowheads).
( full image : 486kb) |
|
|
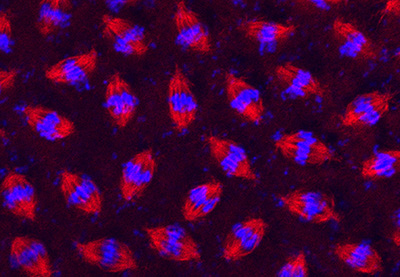
Figure 20:
Synchronous cell division in a syncytial
blastoderm of a developing fruit fly
(Drosophila melanogaster) embryo. After
fertilization, the embryo undergoes an
amazing series of 13 metasynchronous mitotic
divisions to produce nearly 6,000 nuclei in
a matter of a couple of hours. The embryo
was stained with antibody against β-tubulin
(red) to label mitotic spindles and DAPI (blue) to label DNA.
( full image : 352kb) |
|
|
Figure 21:
Localization of CLIC (green), β-tubulin
(red) and DNA (blue) in developing head of a
stage 10 Drosophila embryo: this is a
lateral view with anterior end up, and
ventral at side left. CLIC is highly
concentrated in the apical region of
columnar epithelial cells of the stomodeum
(arrow), which will form the foregut. In
addition, the cell borders (actin-rich
cortex) are outlined by intense CLIC
staining, specifically in metaphase cells
located in mitotic zone 3 (top left), as
well as in mitotic zones 5 and 9, near the
cephalic furrow (middle). Mitotic zones
represent discrete building blocks for
various functional units of the larva,
ultimately serving as a blueprint for the
body plan of the adult fly.
( full image : 1,645kb) |
|
|
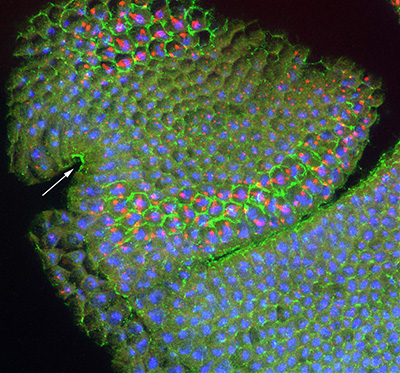
Figure 22:
Lateral view of stage 11 Drosophila embryo
stained for CLIC (green), β-tubulin (red)
and DNA (blue): anterior is left and ventral
is bottom. In addition to prominent CLIC
staining at the plasma membrane of mitotic
cells (look for the large red dots, which
are centrosomes), CLIC is enriched in cells
along the ventral midline as well as the
epithelial cells of the developing tracheal
placodes (asterisks). These placodes will
invaginate as nascent tubes, grow in length,
fuse together, and branch into an
interconnected network of about 10,000
air-filled tubes that allow for gas
transport. This “tree” of interconnected
branching tubes is analogous to the human
lung.
( full image : 67kb) |
|
|
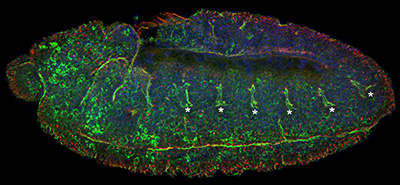
Figure 23:
Dorsal closure: this is the final major
morphogenetic event in Drosophila
embryogenesis. This is a view looking down
on the “back” of an embryo stained for β-tubulin
(green) and actin filaments (red), the two
major cytoskeletal filaments in Drosophila.
The actin filaments form two giant
symmetrical cables, which resemble the eye
of a needle. These cables stitch together
two opposite sides of the epithelium
(“skin”) to fill the red gap (underlying
amnioserosa tissue layer) in a manner a cut
in the skin heals. Defects in this process
during human embryonic development represent
a type of birth defect that involves
malformation of the spinal cord.
( full image : 891kb) |
|
|
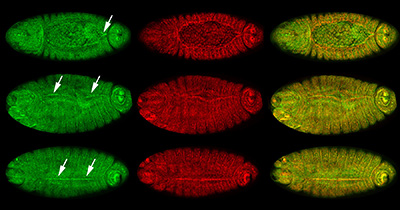
Figure 24:
Localization of CLIC (green) and β-tubulin
(red) at different stages of dorsal closure:
merged images of CLIC and β-tubulin appear
yellow in the right column. Dorsal view
(anterior/head, left) for all embryos. Top,
middle, and bottom rows show high
concentration of CLIC (arrows) at sites of
progressive zippering of opposing epithelial
sheets. This process is analogous to pulling
on strings of a purse, where the strings are
the actin cables and the purse bag is the
epithelium. Note that CLIC is enriched, just
before and after, the epithelium stitches
together.
( full image : 773kb) |
|
|
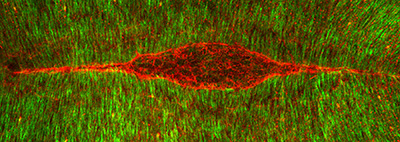
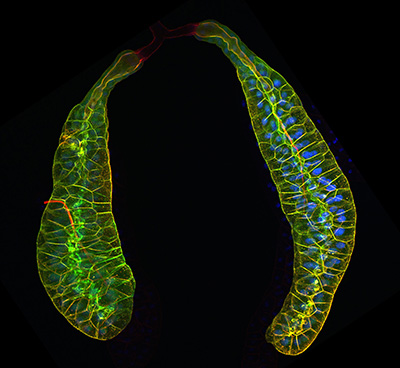
Figure 25:
Pair of third larval instar salivary glands
expressing Lifeact-GFP to visualize actin
filaments (green), and labeled with
phalloidin (red), a separate marker for
actin filaments. At the end of larval
development, these glands secrete a sticky
“glue” that is expectorated to provide a
substrate for subsequent pupal development.
The bulk of the tissue shown is the
glandular component: at the top of the image
is pair of faint, red-stained ducts that
deliver secretory product into a common duct
that empties into the mouth. Credit: Soichi
Tanda (collaborator) https://www.researchgate.net/profile/Soichi_Tanda
( full image : 438kb) |
|

|
Figure 26:
Scanning electron micrograph of an adult fly
head. Prominent features are the eyes, the
antenna, which detects odors and motion, and
the proboscis, an snout-like tubular
appendage used for feeding and sucking. The
compound eye consists of approximately 800
ommatidial units and mechanosensory
bristles. Each ommatidium contains a set of
photoreceptor cells and associated support
cells, including cone cells and pigment
cells.
( full image : 513kb) |
|
|
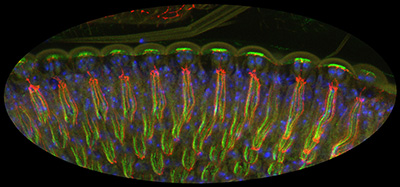
Figure 27:
Lateral view of differentiating
photoreceptors in pupal eye stained for CLIC
(green), armadillo (red), and DNA (blue).
Light would enter the tissue from the top of
the image: the photoreceptor cells are very
tall, and form a channel to capture the
light. The bright green staining at the top of the
image reflects high levels of CLIC in the
microvilli of cone cells: these cells form
the cornea, which acts as a lens. The thin
strands of red and green running
top-to-bottom reveal the localization of CLIC to the rhabdomeres, extraordinary
specializations of the photoreceptor apical
surface that that contain a tightly packed
array of approximately 60,000 microvilli,
serving to enhance capacity for detection of
light.
( full image : 700kb) |
|
|
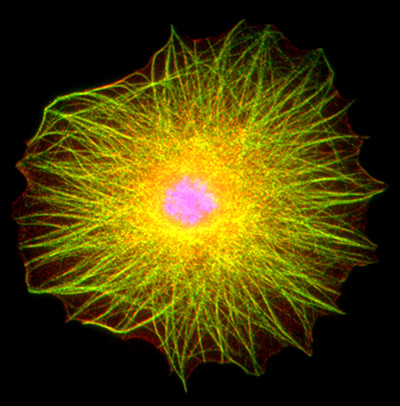
Figure 28:
Drosophila blood cell stained for actin
filaments (red), acetylated α-tubulin
(green), and DNA (blue). This type of blood
cell, a lamellocyte, is part of the insect immune system. It
is a relatively large cell: the nucleus is
located in the center, and a complex network
of overlapping actin filaments and
microtubules gives a yellowish-green
appearance to the cells’ cytoskeleton. In
addition to their close association with
actin filaments, the microtubules often show
conspicuous associations with the plasma
membrane of the cell.
( full image : 475kb) |
|
Heritage College of Osteopathic Medicine
Irvine Hall, Athens, Ohio 45701
740-593-2530 740-597-2778 fax
Contact Information:
(740) 593–9381 | Building 21, The Ridges
Ohio University Contact Information:
Ohio University | Athens OH 45701 | 740.593.1000 ADA Compliance | © 2018 Ohio University . All rights reserved.
