- News & information
- About
- History
- George V. Voinovich
- George V. Voinovich Collection
- Calendar
- How to Find Us
- News
- Archives
- Photojournalism Fellowship Project
- Photo Essays
- Current Fellow
- Previous Fellows
- Reports and Publications
- Archives
- Students
- Prospective
- Center for Entrepreneurship
- Environmental Studies
- HTC/Voinovich School Scholars
- Master of Public Administration
- Current
- HTC/Voinovich School Scholars
- Center for Entrepreneurship
- Environmental Studies
- Master of Public Administration
- Alumni
- Contact
- School Leadership
- Strategic Partners Alliance
- Ohio University Public Affairs Advisory Committee
- Ohio University Public Affairs Advisory Committee
- Faculty and Fellows
- Faculty
- Visiting Professors
- Voinovich Fellows
- Professional Staff
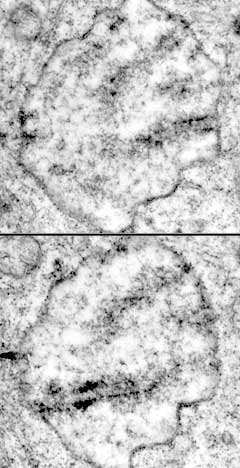
Meiosis & Karyotyping for Plasmodiophorids
Personal Comments
Some of the first images we obtained of Sorosphaera veronicae when I started TEM studies of it back in the early 70s showed synaptonemal complexes (SCs) . I was familiar with SCs because my dissertation at Iowa State University was on meiosis in Lilium longiflorum . SCs in Sorosphaera led us to suspect that the noncruciform divisions in transitional sporogenic plasmodia were meiosis. One of my goals was to see how many plasmodiophorids had meiosis in transitional sporogenic plasmodia, and if they did, use the SCs to karyotype them. Since the nuclei of plasmodiophorids are only several micrometers in diameter, conventional light microscopy was insufficient for counting chromosomes. We thought that using serial sections as shown by Moens and Perkins (1969) was the way to get karyotypes.
Suzanne Harris was a MS student in the department who showed the type of perseverance needed to take on the difficult assignment of serial sectioning entire nuclei (no skips were allowed) of Sorosphaera in our first attempt to get an accurate karyotype for a plasmodiophorid. I happened to be on a sabbatical in 1978-79 in Michael Bennett's lab at the then Plant Breeding Institute in Trumpington, Cambridge, UK, where they were serial sectioning entire nuclei of wheat, which are magnitudes larger than nuclei of plasmodiophorids. Suzanne visited me over the Christmas holidays in 1978 and we worked on interpreting her serial sections of Sorosphaera . Her MS thesis was the first accurate karyotype of a plasmodiophorid.
When I returned to Ohio University after my sabbatical, I was able to apply the techniques I learned from Jim Smith in Bennett's lab to the plasmodiophorids. A summary of the method that was based on Wells (1974) and Rowley and Moran (1975) was published in Zoosporic Fungi in Teaching and Research , edited by M.S. Fuller and A. Jaworski. Although the digitizing pad and software we used for measuring the synaptonemal complexes are now way out-of-date, what I did that was special was to make serial sections of specific, individual infected cells. I would take a 1/2 µm "thick" section and look at it through light microscopy. When I found an infected cell that had a stage of the plasmodiophorid that I wanted to serial section, I would orient the block from which the thick section was taken in such a way that when viewed with a dissecting microscope, the cells could be seen in the freshly cut surface. I would then trim to the cell I wanted, reposition the block back onto the ultramicrotome, and make serial sections .
Analyzing the serial sections was not as straight forward as it seems when reading the publications. For the SCs in plasmodiophorids the sections needed to be 100-130 nm (gold). If they were thinner (silver), which many microscopists would recommend for improved resolution at higher magnifications, the SCs could be lost when viewed laterally. Also, if one or more ends of SCs did not end at the nuclear envelope in a nucleus that had been serial sectioned, the analysis could not be completed. There were a lot of nuclei that we serial sectioned, but could not be used in the analysis. One interesting aspect of plasmodiophorid meiotic nuclei is how the SCs end near the poles in a double bouquet. The poles are defined in pachynema by centrioles, and the SCs end on the nuclear envelope near the centrioles. This arrangement became clear to us when we started making models of the nuclei . With today's advancements in computer and software technology the computer could make the 3D reconstructions and a 3D printer could be used to build the models.
The idiogram of the 20 chromosomes of P. brassicae based on serial sections of synaptonemal complexes was supported by studies that of telomere-to-telomere genome assembly (Javed et al 2024) and contigs in the nuclear genome (Stjelja et al 2019) that included idiograms of the P. brassicae genome similar to the idiogram of P. brassicae summarized in Idiograms based on synaptonemal complexes .
Images of Meiosis, Synaptonemal Complexes, & Karyotyping
- Serial sections
- Sorosphaera synaptonemal complexes, TEMG
- Membranosorus synaptonemal complex, TEMG
- Membranosorus synaptonemal complexes, TEMG
- Plasmodiophora sporogenic transitional plasmodium, TEMG
- Sorodiscus survey of pachynema, TEMG
- Sorodiscus synaptonemal complex, TEMG
- Plasmodiophora synaptonemal complexes, TEMG
- Pseudoligniera transitional sporogenic plasmodium, TEMG
- Spongospora transitional sporogenic plasmodium, TEMG
- Polymyxa sporogenic transitional plasmodium, TEMG
- Polymyxa transitional nucleus with synaptonemal complexes, TEMG
- Polymyxa serial sections of synaptonemal complex, TEMG
- Tetramyxa serial sections of synaptonemal complex, TEMG
- Tetramyxa synaptonemal complexes, TEMG
- Woronina transitional sporogenic plasmodium, TEMG
- Plasmodiophora metaphase I & II, TEMG
- Idiograms based on synaptonemal complexes
References for Meiosis, Synaptonemal Complexes, & Karyotyping
- Braselton, J. P. 1982. Karyotypic analysis of Plasmodiophora brassicae based on serial thin sections of pachytene nuclei. Can. J. Bot. 60: 403-408.
- _____. 1983. Karyotypic analysis of Polymyxa betae (Plasmodiophoromycetes) based on serial thin sections of pachytene nuclei. Can. J. Bot. 61: 3202-3206.
- _____. 1984. Karyotypic analysis of Polymyxa graminis (Plasmodiophoromycetes) based on serial sections of synaptonemal complexes. Can. J. Bot. 62: 2414-2416.
- _____. 1988. Karyotypic analysis of Ligniera verrucosa (Plasmodiophoromycetes). Can. J. Bot. 67: 1216-1218.
- _____. 1988. Karyotypic analysis of Membranosorus heterantherae (Plasmodiophoromycetes). Can. J. Bot. 67: 1219-1220.
- _____. 1990. Ultrastructural karyology of Tetramyxa parasitica (Plasmodiophoromycetes). Can. J. Bot. 68: 594-598.
- _____. 1992. Ultrastructural karyology of Spongospora subterranea (Plasmodiophoromycetes). Can. J. Bot. 70: 1228-1233.
- _____ & D. P. Dylewski. 1986. Karyotypic analysis of Woronina pythii . Mycologia 78: 511-513.
- _____ & F. T. Short. 1985. Karyotypic analysis of Plasmodiophora diplantherae . Mycologia 77: 940-945.
- Dylewski, D. P. & C. E. Miller. 1984. The ultrastructure of meiosis in Woronina pythii (Plasmodiophoromycetes). Mycologia 76: 1075-1088.
- Garber, R. C. & J. R. Aist. 1979. The ultrastructure of meiosis in Plasmodiophora brassicae (Plasmodiophorales). Can. J. Bot. 57: 2509-2518.
- Harris, S. E., J. P. Braselton, & C. E. Miller. 1980. Chromosomal number of Sorosphaera veronicae (Plasmodiophoromycetes) based on ultrastructural analysis of synaptonemal complexes. Mycologia 72: 916-925.
- Javed, M. A. et al . 2024. Telomere-to-telomere genome assembly of the clubroot pathogen Plasmodiophora brassicae . Genome Biol. Evol. 16 Doi: 10.1093/gbe/evae122
- Moens, P. B. & F. O. Perkins. 1969. Chromosome number of a small protist: accurate determination. Science 166: 1289-1291.
- Rowley, J. C. III & D. T. Moran. 1975. A simple procedure for mounting wrinkle-fee sections on formvar-coated slot grids. Ultramicroscopy 1: 151-155.
- Stjelja, S., J. Fogelqvist, C. Tellgren-Roth & C. Dixelius. 2019. The architecture of the Plasmodiophora brassicae nuclear and mitochondrial genomes. Scientific Reports 9:15753 Doi: 10.1038/s41598-019-52274-7
- Wells, B. 1974. A convenient technique for the collections of ultra-thin sections. Micron 5: 79-81.
Contact Information:
(740) 593–9381 | Building 21, The Ridges
Ohio University Contact Information:
Ohio University | Athens OH 45701 | 740.593.1000 ADA Compliance | © 2018 Ohio University . All rights reserved.
