- News & information
- About
- History
- George V. Voinovich
- George V. Voinovich Collection
- Calendar
- How to Find Us
- News
- Archives
- Photojournalism Fellowship Project
- Photo Essays
- Current Fellow
- Previous Fellows
- Reports and Publications
- Archives
- Students
- Prospective
- Center for Entrepreneurship
- Environmental Studies
- HTC/Voinovich School Scholars
- Master of Public Administration
- Current
- HTC/Voinovich School Scholars
- Center for Entrepreneurship
- Environmental Studies
- Master of Public Administration
- Alumni
- Contact
- School Leadership
- Strategic Partners Alliance
- Ohio University Public Affairs Advisory Committee
- Ohio University Public Affairs Advisory Committee
- Faculty and Fellows
- Faculty
- Visiting Professors
- Voinovich Fellows
- Professional Staff
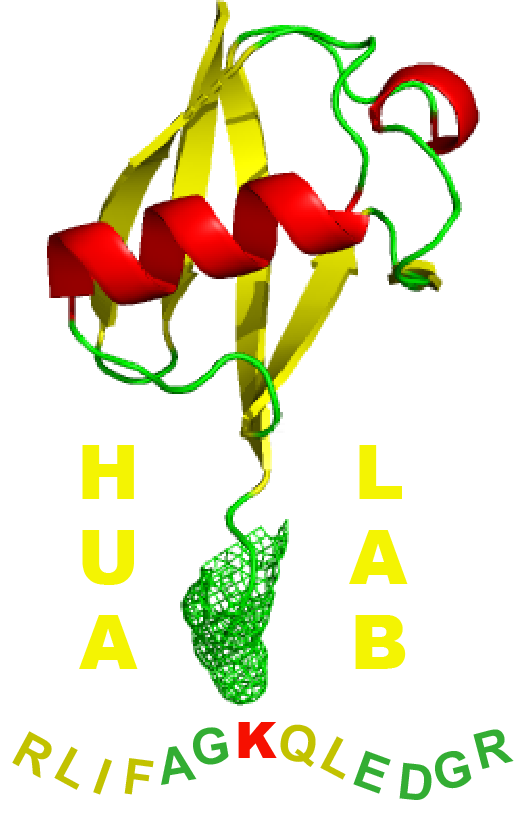
Identification of major lysine residues of S(3)-RNase of Petunia inflata involved in ubiquitin-26S proteasome-mediated degradation in vitro

Hua, Z., and Kao, T.H.
Abstract
S-RNase-based self-incompatibility has been identified in three flowering plant families, including the Solanaceae, and this self/non-self recognition mechanism between pollen and pistil is controlled by two polymorphic genes at the S -locus, S-RNase and S-locus F-box ( SLF ). S-RNase is produced in the pistil and taken up by pollen tubes in a non- S -haplotype-specific manner. How an allelic product of SLF interacts with self and non-self S-RNases to result in growth inhibition of self pollen tubes is not completely understood. One model predicts that SLF targets non-self S-RNases for ubiquitin/26S proteasome-mediated degradation, thereby only allowing self S-RNase to exert cytotoxic activity inside a pollen tube. To test this model, we studied whether any of the 20 lysine residues in S3-RNase of Petunia inflata might be targets for ubiquitination. We identified six lysines near the C-terminus for which mutation to arginine significantly reduced ubiquitination and degradation of the mutant S3-RNase, GST:S3-RNase (K141–164R) in pollen tube extracts. We further showed that GST:S3-RNase (K141–164R) and GST:S3-RNase had similar RNase activity, suggesting that their degradation was probably not caused by an ER-associated protein degradation pathway that removes mis-folded proteins. Finally, we showed that PiSBP1 (P. inflata S-RNase binding protein 1), a potential RING-HC subunit of the PiSLF (P. inflata SLF)-containing E3-like complex, could target S-RNase for ubiquitination in vitro . All these results suggest that ubiquitin/26S proteasome-dependent degradation of S-RNase may be an integral part of the S-RNase-based self-incompatibility mechanism.

Contact Information:
(740) 593–9381 | Building 21, The Ridges
Ohio University Contact Information:
Ohio University | Athens OH 45701 | 740.593.1000 ADA Compliance | © 2018 Ohio University . All rights reserved.