- News & information
- About
- History
- George V. Voinovich
- George V. Voinovich Collection
- Calendar
- How to Find Us
- News
- Archives
- Photojournalism Fellowship Project
- Photo Essays
- Current Fellow
- Previous Fellows
- Reports and Publications
- Archives
- Students
- Prospective
- Center for Entrepreneurship
- Environmental Studies
- HTC/Voinovich School Scholars
- Master of Public Administration
- Current
- HTC/Voinovich School Scholars
- Center for Entrepreneurship
- Environmental Studies
- Master of Public Administration
- Alumni
- Contact
- School Leadership
- Strategic Partners Alliance
- Ohio University Public Affairs Advisory Committee
- Ohio University Public Affairs Advisory Committee
- Faculty and Fellows
- Faculty
- Visiting Professors
- Voinovich Fellows
- Professional Staff
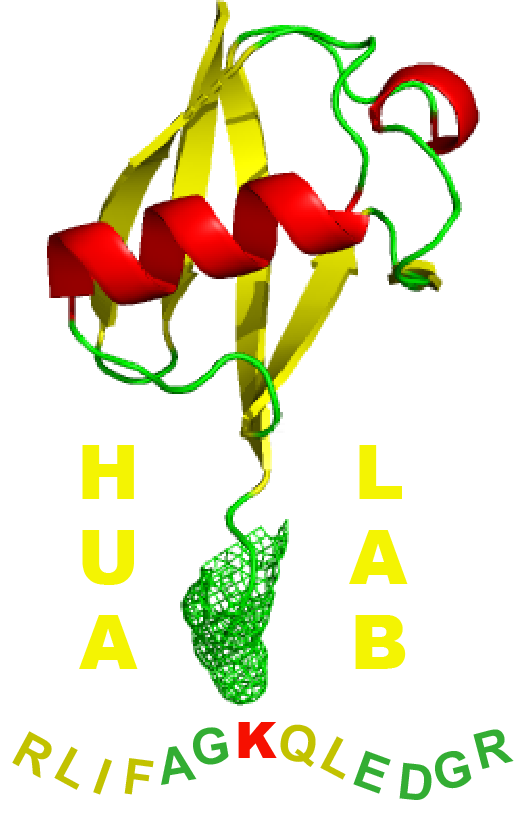
The ubiquitin-26S proteasome system and autophagy relay proteome homeostasis regulation during silique development

Yu, P.(g) and Hua, Z.*
Abstract
Functional studies of the ubiquitin-26S proteasome system (UPS) have demonstrated that virtually all aspects of the plant's life involve UPS-mediated turnover of abnormal or short-lived proteins. However, developmental characterization of the UPS, including in seeds and fruits, remains scarce although mutants of its several core elements are embryonically lethal. Unfortunately, early termination of embryogenesis limits the scope for characterizing the UPS activities in reproductive organs. Given both economic and societal impact of reproductive production, such studies are indispensable. Here, we systematically compared expression changes of multiple 26S proteasome subunits along with the dynamics of proteasome activity and total protein ubiquitylation in seedlings, developing siliques or embryos of Arabidopsis thaliana . Since autophagy plays the second largest role in maintaining proteome stability, we parallelly studied three rate-limiting enzymes that are involved in autophagy flux. Our experiments unexpectedly discovered that, in opposite to the activities in seedlings, both protein and transcript levels of six selected 26S proteasome subunits gradually decline in immature siliques or embryos toward maturation while the autophagy flux rises albeit in a nutrient-rich condition. We also discovered a reciprocal turnover pathway between the proteasome and autophagy. While the autophagy flux is suppressed in seedlings by UPS-mediated degradation of its three key enzymes, transcriptional reprogramming dampens this process in siliques that in turn stimulates a bulk autophagic degradation of proteasomes. Collectively, our discovery about the developmental changes of the UPS and autophagy activities suggests that they relay the proteome homeostasis regulation in early silique and/or seed development highlighting their developmental interactions.

Contact Information:
(740) 593–9381 | Building 21, The Ridges
Ohio University Contact Information:
Ohio University | Athens OH 45701 | 740.593.1000 ADA Compliance | © 2018 Ohio University . All rights reserved.