Research

We are studying how osteoarthritis (OA) occurs with different risk factors, such as aging and obesity, with particular interests in studying the role of cellular metabolism in OA pathogenesis.
Our recent findings demonstrate that one type of protein post-translational modification-lysine malonylation (MaK) is involved in chondrocyte metabolic dysfunctions during aging and obesity. We are studying how MaK is dysregulated and testing if MaK is good therapeutic target for OA. Another line of research is focused on studying the role of growth hormone (GH) in chondrocyte metabolism and OA development during aging.
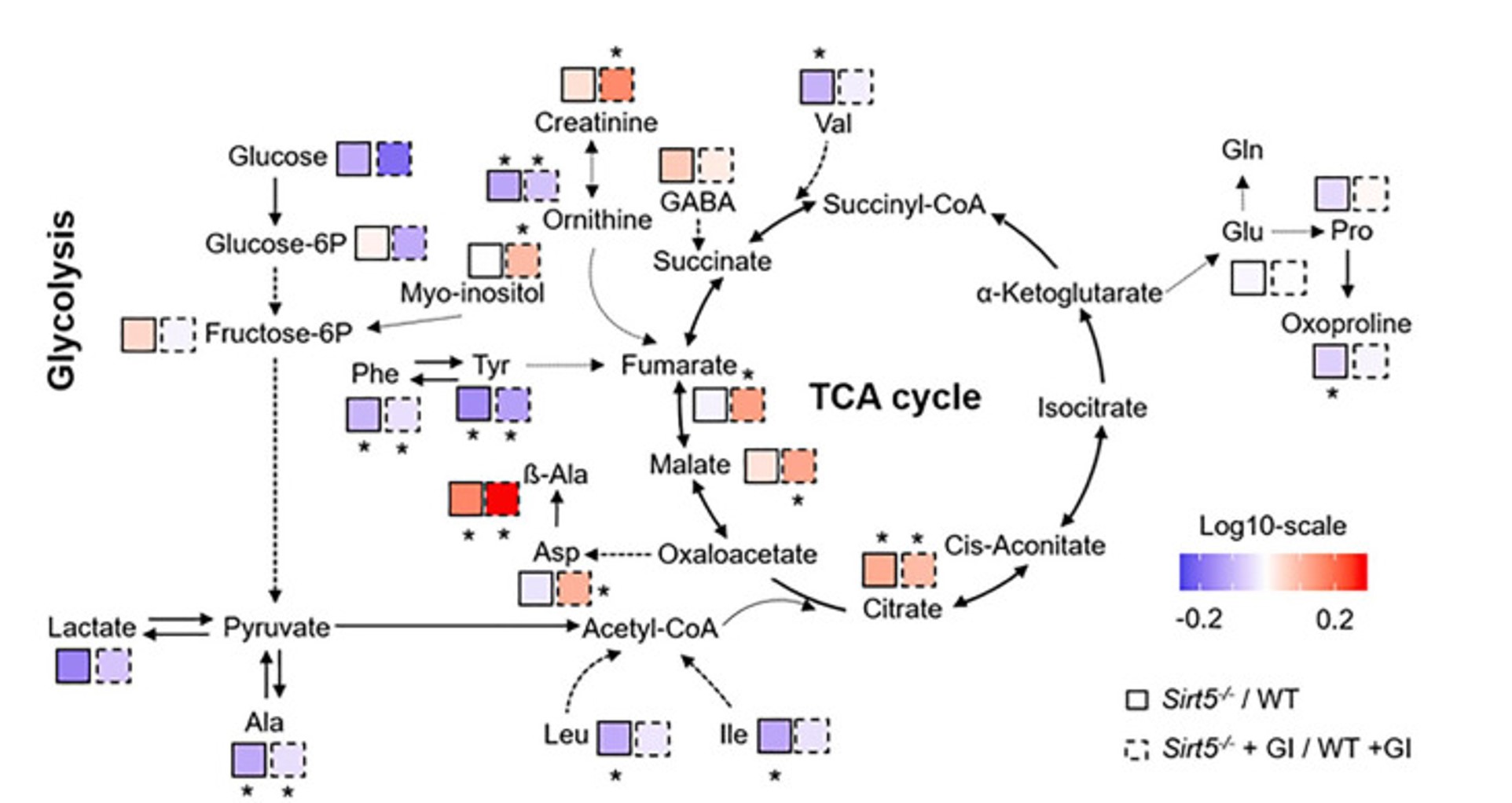
Sirt5 and protein malonylation in metabolism and OA
Chondrocyte lipogenesis and OA
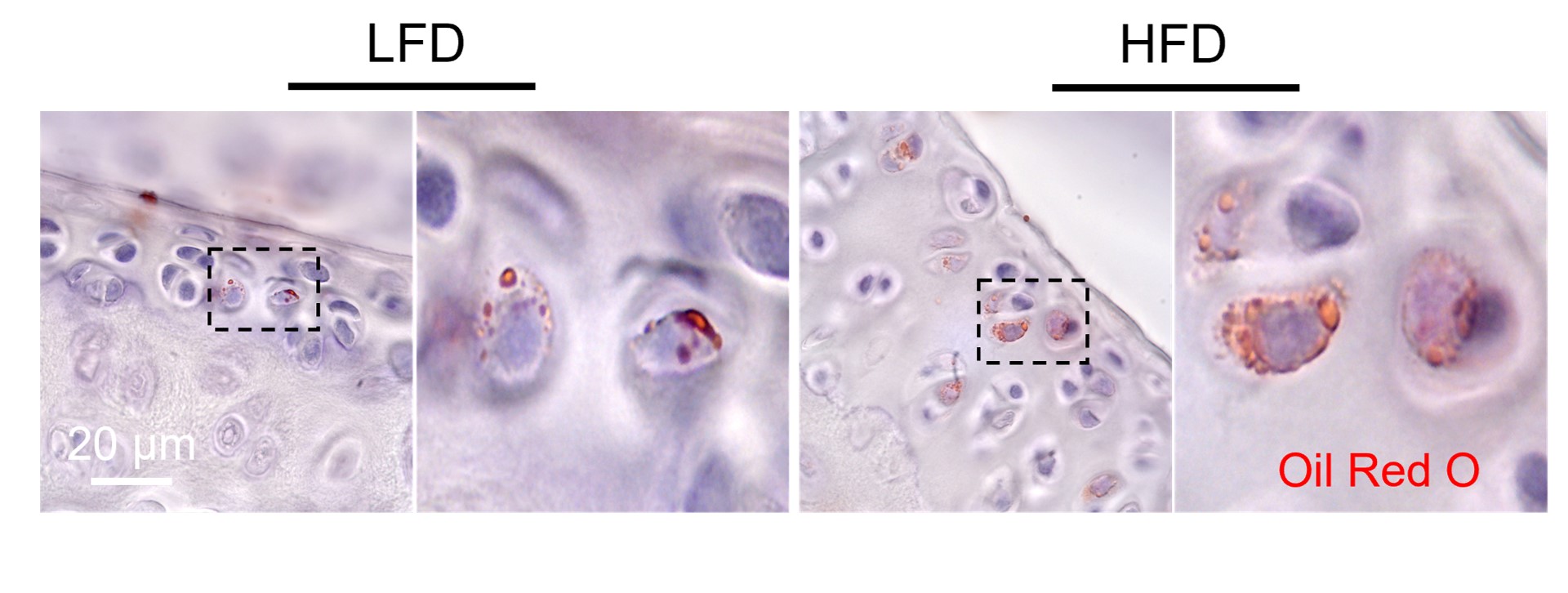
Chondrocytes naturally contain lipid droplets. The amount of cartilaginous lipid often times positively correlated with OA severity. It is unknown how the lipids were accumulated. Previous studies suggested chondrocytes can uptake fat from surroundings but overlooked another important source---de novo lipogenesis. Our previous work showed that there is increased expression of acetyl-CoA carboxylase 1 (ACC1), an essential enzyme during the first committed step toward FA synthesis, in cartilage during obesity. In this project, we are investigating how ACC1 mediated de novo lipogenesis plays a role in joint inflammation and OA.
Growth hormone (GH) in chondrocyte metabolism and OA
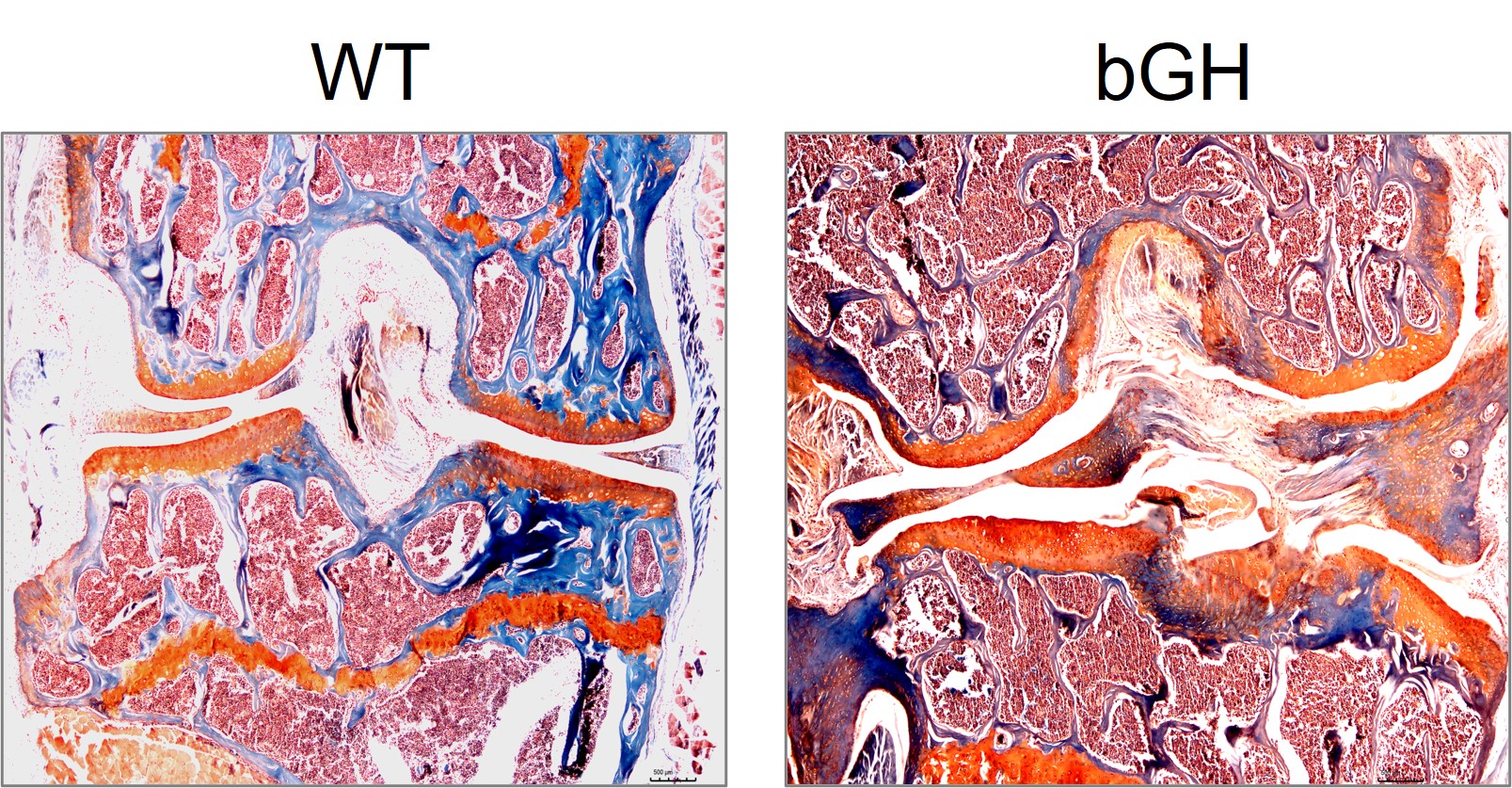
Nearly 90% of patients with acromegaly, a hormonal disorder with an excessive amount of GH production due to a GH secreting pituitary adenoma, have joint issues. Our previous research showed that mice with over expression of bovine GH (bGH) have accelerated OA development, which mice with expression of GH receptor antagonist (GHa) are protected from developing aging associated OA. In this project, we are investigating how GH is playing a role in the joint disease and in regulating chondrocyte metabolism. We are also investigating if GH can be a therapeutic target for OA.
Laboratory Collaborations
Our current collaborators include: John Kopchick (OU), Dan Raftery (U of Washington), Martin-Paul Agbaga (OUHSC), Martin Lotz (Scripps), Mick Jurynec (U of Utah), Anne-Marie Malfait (Rush) and many more.


